Overview of Alzheimer’s Disease
Alzheimer’s Disease (AD) is a prevalent and progressive brain disorder that primarily affects memory, thinking, and behavior. It stands as the leading cause of dementia, a term encompassing a cluster of symptoms that significantly impede an individual’s daily life and activities. One of the most crucial points to emphasize is that Alzheimer’s is not a natural consequence of aging, and as of now, there is no known cure. Nevertheless, there are treatments available to help manage its symptoms and enhance the quality of life for those affected by AD and their caregivers. The percentage of people with Alzheimer’s dementia increases dramatically with age. Five percent of people aged 65 to 74, 13.1% of people aged 75 to 84, and 33.3% of people aged 85 or older have Alzheimer’s dementia. This underscores the fact that AD predominantly affects older individuals.
Alzheimer’s Disease is characterized by a gradual deterioration of memory and cognitive abilities, ultimately rendering individuals unable to perform even the simplest of tasks. The disease is marked by distinct changes in the brain, leading to the buildup of specific proteins. Over time, AD causes the brain to shrink and the gradual death of brain cells. As a result, it manifests as a relentless decline in memory, thinking, behavior, and social skills, all of which are hallmarks of dementia. The initial signs of AD may include mild memory lapses, such as forgetting recent events or conversations. As the disease progresses, these memory problems become more severe, coupled with the emergence of additional symptoms like confusion, disorientation, and even getting lost in familiar surroundings. Diagnosing AD can be challenging due to its slow progression. A comprehensive evaluation by a specialist is often necessary, involving an in-depth assessment of symptoms and potentially further diagnostic tests such as brain scans.
Several risk factors have been associated with AD, including increasing age, a family history of the disease, untreated depression, and factors linked to cardiovascular disease. Although complete prevention strategies remain elusive, maintaining a healthy lifestyle and effectively managing cardiovascular risk factors are believed to reduce the risk of developing AD. Some studies also suggest that cognitive stimulation, social engagement, and both physical and mental exercise may have a protective effect.
The problem: gamma oscillations and PV cells
AD is a complex neurodegenerative disorder characterized by the accumulation of abnormal proteins and disruptions in neural activity. Two key proteins associated with AD are amyloid beta (Aβ) and phosphorylated tau (p-tau), which are implicated in the pathogenesis of the disease. Furthermore, alterations in the brain’s oscillatory activity, particularly in the gamma frequency range, are causally linked to the cognitive decline observed in AD.
The dysregulation of gamma activity has been further linked to pathologic network hyperexcitability in animal models of AD. Recent research has revealed that exogenously induced 40 Hz gamma oscillations can reduce the abnormal protein levels in a mouse model of AD. Furthermore, in pre-symptomatic AD mice, the induction of gamma activity appears to prevent subsequent neurodegeneration and behavioral deficits, suggesting that gamma oscillations may offer a powerful new therapeutic approach. Recent evidence points to specific alterations in high-frequency activity within the gamma band as a result of dysfunction in GABAergic parvalbumin+ (PV+) inhibitory interneurons. These PV neurons play a crucial role in generating gamma oscillations, which are essential for information processing and memory formation. However, in AD, PV neurons are impaired, resulting in reduced gamma power, network imbalance, and cognitive decline. Amyloid beta and tau proteins are known to accumulate abnormally in the brains of individuals with AD, and these proteins can impair the function and survival of PV cells.
The promising news is that restoring or mimicking PV neuron function has shown the potential to rescue network function, reduce amyloid pathology, and improve memory performance in animal models of Alzheimer’s disease. This demonstrates the critical role of PV cells in maintaining proper neural network dynamics and cognitive function.
The solution: tACS
While AD remains a formidable challenge with no definitive cure in sight, there are treatments available, both pharmacological and non-pharmacological, aimed at alleviating its symptoms. Among the non-pharmacological approaches, one shining star on the horizon is Transcranial Alternating Current Stimulation (tACS).
AD is characterized by the loss of gamma oscillations, which are essential for proper cognitive function, particularly memory. However, the potential for tACS to entrain these crucial gamma oscillations offers a beacon of hope. Recent studies have shown that tACS at 40Hz, the gamma frequency, holds the promise to restore cognitive abilities and improve memory in individuals affected by AD. In healthy individuals, tACS at 40Hz has demonstrated a remarkable ability to modulate gamma activity in the brain, resulting in cognitive enhancements. This discovery is a foundation for exploring tACS as a potential therapeutic tool for AD. Recent research has extended these findings to AD patients. A valuable dataset collected from mild to moderate AD patients undergoing multisession, multisite tACS treatment has shown promising results. Repeated sessions of 40 Hz tACS have indicated the potential to regulate gamma power and improve cognitive function, particularly in the memory domain.
The Neurotwin project
While tACS at 40Hz holds the promise to restore cognitive abilities and improve memory in individuals with AD, the path forward is illuminated by cutting-edge research and projects like Neurotwin. The vision of Neurotwin is grounded in the belief that personalized brain models can unlock novel neuroscience insights, reduce uncertainty in diagnosis, and serve as the foundation for groundbreaking therapeutic breakthroughs. This vision is underpinned by the idea of capturing individual biophysical and physiological characteristics.
In a world where neuropsychiatric disorders rank as a leading cause of global disability-adjusted life years, solutions remain scarce. However, Neurotwin proposes that digital twins, tailored to each individual, could be central to progress in some cases. These models unite the physics of electromagnetism with physiology, and they are poised to play a fundamental role in understanding and optimizing the effects of stimulation at the individual level.
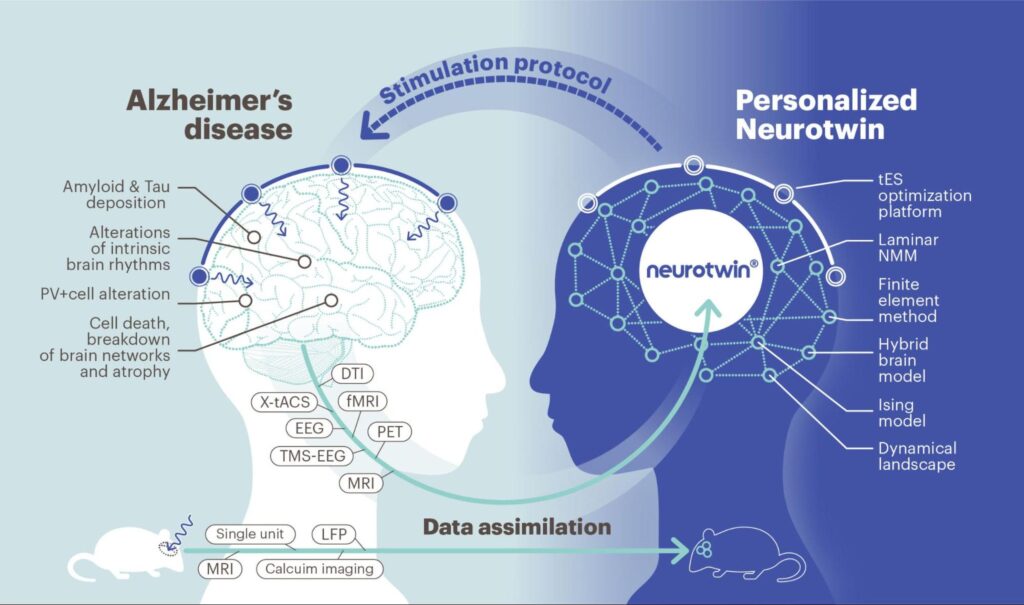
The ambition of Neurotwin is to deliver disruptive solutions by employing model-driven, individualized therapy. The project is developing a comprehensive computational framework that weaves together various scales and levels of detail. This framework will represent the intricate mechanisms of interaction between electric fields and brain networks, assimilating neuroimaging data. With this framework, Neurotwin characterizes the dynamical landscape of individual brains, allowing for the definition of strategies to restore healthy dynamics. Drawing from extensive databases of healthy and AD individuals, the project is creating the first human and rodent NeTs, predicting the effects of stimulation on dynamics.
Neurotwin is collecting detailed multimodal measurements in mice and humans to enhance the predictive power of local and whole-brain models under the influence of electrical stimulation. These findings have been translated into a technology pipeline for designing new personalized neuromodulation protocols, which will be rigorously tested in a cohort of AD patients and healthy controls in randomized double-blinded studies. There is an ongoing Neurotwin clinical pilot, where patients are being stimulated daily for 2 months at home with our Starstim home product.

Conclusion
In the face of Alzheimer’s daunting challenges, promising horizons emerge through groundbreaking research and cutting-edge therapies. As we unravel the intricate web of neural networks and harness the power of gamma oscillations, we draw nearer to a future where personalized brain models and individualized therapies offer new possibilities. With determination and innovation, we move forward, bringing hope and brighter days to those impacted by Alzheimer’s.
Resources and References:
- (2023), 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement., 19: 1598-1695. https://doi.org/10.1002/alz.13016
- Iaccarino, H.F. et al. (2016) Gamma frequency entrainment attenuates amyloid load and modifies microglia, Nature News. Available at: https://www.nature.com/articles/nature20587
- https://www.neurotwin.eu/